Preparation of Equipment for the Realisation of Water Triple Point Cells YouTube
Triple Point of Water The Temperature Where All Three Phases Coexist NA Eye
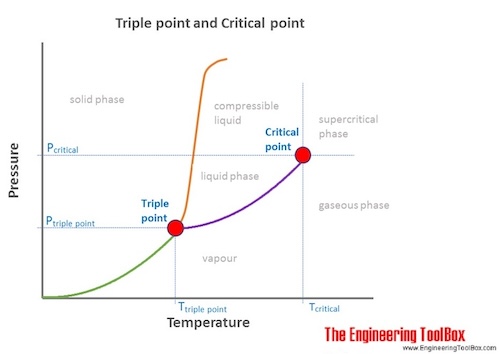
A typical phase diagram.The solid green line applies to most substances; the dashed green line gives the anomalous behavior of water. In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium. It is that temperature and pressure at which the sublimation, fusion.

Thermodynamics Explaining the Triple Point YouTube
This experiment demonstrates the triple point of a substance. Watch how water behaves at the triple point where it co-exists in solid, liquid and vapour form.
[DIAGRAM] Test Point Diagram 161.35.132.113
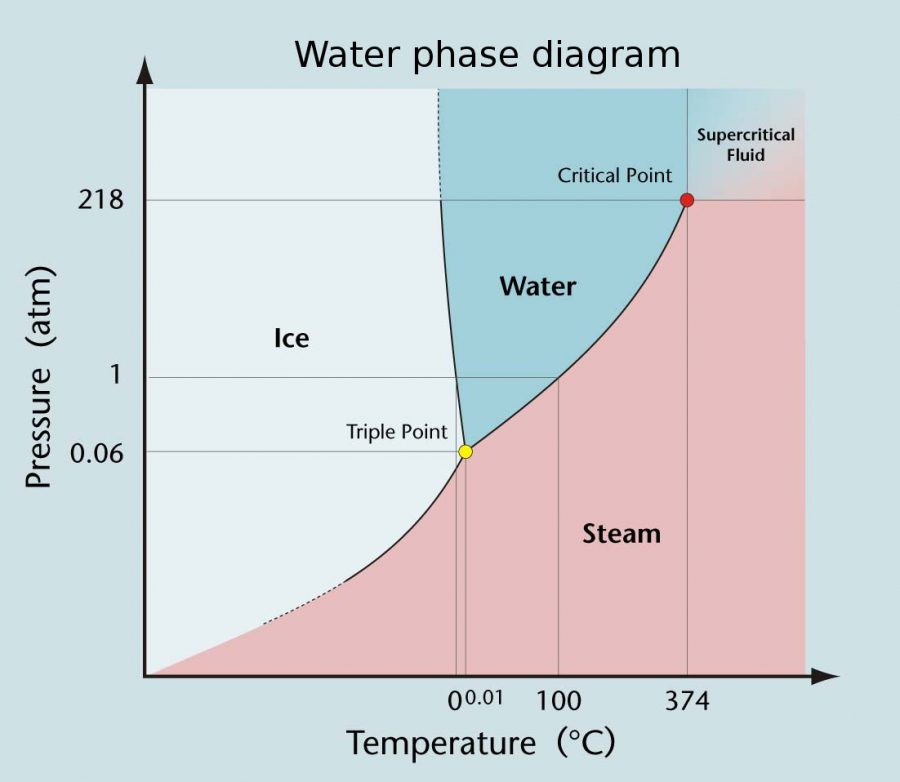
You will be thrilled to know that pure water has a triple point at 0.01°C temperature, which is just above the freezing point. The pressure at this point remains at 611.5 Pa. While the triple point temperature is known as T3, the triple point pressure is denoted by P3. Courtesy : UC Santa Cruz Physics Demonstration Lab.

The P − T phase diagram of water system near its triple point (TP).... Download Scientific Diagram
Triple point of water is 273 K temperature and 0.46 cm of mercury pressure. The triple point of water, T 3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of water and absolute zero.

Why use a triple point of water? Namibian Mining News
Triple point of water. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in equilibrium. It is a specific case of thermodynamic phase equilibrium. The term "triple point" was coined by James Thomson in 1873.

The Universe of Discourse A message to the aliens, part 10/23 (temperature)
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist.

Preparation of Equipment for the Realisation of Water Triple Point Cells YouTube
The triple point of any substance is that temperature and pressure at which the material can coexist in all three phases (solid, liquid and gas) in equilibrium. Specifically the triple point of water is 273.16 K at 611.2 Pa. Ask the experts your physics and astronomy questions, read answer archive, and more.

Triple Point Phase Diagram of water YouTube
Difference between Boiling point and Triple point. In the case of a triple point, all the states of matter exist while in the case of a boiling point only two states of matter exist. It is impossible to drop the boiling point below the triple point. Suggest Corrections. 6.

Triple Point of Water YouTube
This paper is a part of guidelines, prepared on behalf of the Consultative Committee for Thermometry, on the methods how to realize the International Temperature Scale of 1990. It discusses all major issues linked to the triple point of water when used as a fixed point for the realization of the kelvin. 1. Introduction.

What is Tripple point? PhysicStuff
Examples of Triple Point in Nature. One example of a triple point in nature is the triple point of water. At this point, water can exist in all three states: ice, liquid, and vapor. The triple point of water occurs at a temperature of 0.01°C and a pressure of 611.73 pascals. This is why, at high altitudes, water can boil at a lower temperature.

Triple Point Definition Triple Point of Water
The triple point of water, T 3 = 273.16 K, is the standard fixed-point temperature for the calibration of thermometers. This agreement also sets the size of the kelvin as 1/273.16 of the difference between the triple-point temperature of the water and absolute zero. The phase diagram of water is a pressure-temperature diagram for water that.

science chemistry experiment states of matter Fundamental Photographs The Art of Science
The triple point occurs where the solid, liquid, and gas transition curves meet. The triple point is the only condition in which all three phases can coexist, and is unique for every material. Water reaches its triple point at just above freezing (0.1° C) and at a pressure of 0.006 atm.

Triple Point of Water YouTube
eCHEM 1A: Online General ChemistryCollege of Chemistry, University of California, Berkeleyhttp://chemistry.berkeley.edu/echem1aCurriculum and ChemQuizzes dev.
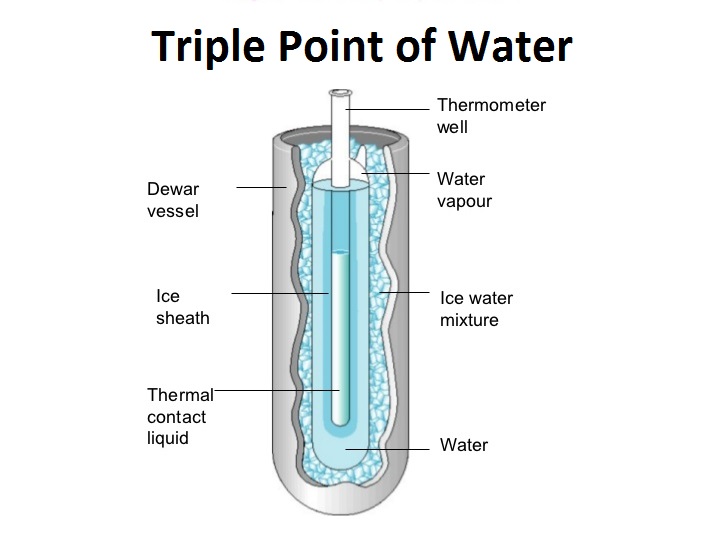
The Triple Point of Water Coexistence of Solid Liquid and Gas
The triple point of water is at 273.16 kelvin (0.01 °C or 32.02 °F) and a pressure of 611.7 pascals (6.117 millibars, 0.0060373057 atm). (Some sources list a pressure of 611.73 Pa, while others cite a pressure of 611.657 Pa.) At this point, ice, water, and water vapor coexist in stable form. The tiniest change in temperature or pressure.

science chemistry experiment states of matter Fundamental Photographs The Art of Science
The triple point of water is a unique and fascinating concept in thermodynamics. It is the specific temperature and pressure at which water can exist in all three states simultaneously: solid, liquid, and gas. At this point, the water molecules are in perfect equilibrium, with the same number of molecules transitioning between each state.The triple point of water occurs at a temperature of 0..

Triple point of water analogy. intensive variables critical... Download Scientific
The triple point of water corresponds to the minimum pressure at which water in the liquid state can exist. At pressures below the triple point (as in outer space), solid ice when heated at constant pressure is converted directly into water vapor in a process known as sublimation. In general, sublimation is a phase change of a substance.